Description
UScreen™ Rapid Urine Drug Test Cup, Fourteen (14) Panel Drug Screening Test Cup with 5 Adulterants, FDA OTC Approved, CLIA Waived, USSCUPA-14CLIA
Exciting News: The UScreen Cup is Back! Say goodbye to the long wait times for drug test results! Introducing UScreen™ – your go-to for fast, reliable drug testing. With our innovative UScreen™ Urine Drug Test Cup, you'll receive negative results with a clear, solid line in just 90 seconds or less. Our comprehensive UScreen™ 14 Panel Urine Drug Test Kit covers testing for 14 drugs of abuse. Plus, rest assured knowing that UScreen™ is both Over-the-Counter and CLIA Waived FDA Cleared, ensuring accuracy and peace of mind. Exclusive distribution by TransMed Company guarantees quality and accessibility.
This Highest Quality drug test cup is an easy, fast, qualitative method for screening without the need of instrumentation. The Uscreen™ Rapid Drug Test Cup is a single step, fully integrated, self contained cup used for the simultaneous, qualitative detection of multiple drug metabolites at specific cutoff levels in human urine. No tipping or turning required for activation and results can be read in 5 minutes.
New And Improved Features
★ Results Remain Valid for 60 Minute Read Time
★ Click Seal Cap - Insures Cap is Securely Tightened
★ Control & Test Lines are 10% Wider Than the Leading Competitors
UScreen Features & Benefits
- Easier to Handle Full Size 4-Sided Cup
- CLIA Waived Drug Test
- Over-the-Counter (OTC) Cleared by the FDA
- Sold in boxes of 25 tests
- 99% Accuracy Rate
- Adheres to the SAMHSA Cutoff Levels
- Easy to Read Results in 5 minutes—results are stable for 60 minutes
- Easy one-step operation
- Zero exposure to donor specimen
- Donor friendly cup opening
- Fast Dark Test lines
- Most results within 60-90 seconds!
- Built in Adulteration Test / Specimen Validity Tests (SVT)—helps to monitor specimen tampering
- Dual scale °F/°C Temperature Strip verifies if urine is "fresh" and not diluted
- Self-contained cup is ideal for sending preliminary positive specimens to the lab for confirmation
- Simple to use, no need to tilt cup to activate test—read results at 5 minutes
UScreen Product Information & Reference Materials
 UScreen Drug Test Cup Brochure
UScreen Drug Test Cup Brochure
 UScreen Drug Test Cup Package Insert (OTC Version)
UScreen Drug Test Cup Package Insert (OTC Version)
 UScreen Drug Test Cup Package Insert (Full CLIA Version Including Performance Characteristics)
UScreen Drug Test Cup Package Insert (Full CLIA Version Including Performance Characteristics)
| 14 Panel Configuration with Adulteration | |||
| Item Number | Drugs Screened | Adulterants Screened | Regulatory Approvals |
| USSCUP14CLIA | AMP1000, BAR300, BUP10, BZO300, COC300, MDMA500, mAMP1000, MTD300, OPI300, OXY100, PCP25, PPX300, TCA1000, THC50 | CR - Creatinine SG - Specific Gravity PH - pH Level NI - Nitrates GL - Glutaraldehyde |
FDA Over-the-Counter (OTC) CLIA Waived |
Performing the Test
- Remove the test cup from the foil pouch by tearing at the notch and use it as soon as possible. Open the cap of the test cup and void directly into the test cup to reach the minimum urine level.
- Verify that temperature is within range on the temperature strip (90° – 100°F).
- To activate test, place the screw-top lid on the cup and turn until tight.
- Remove label. For cups with specimen validity tests (adulteration controls), compare each of the specimen validity test pads with the corresponding color blocks on the enclosed color chart. If pad colors are outside normal range, open a second test cup, collect a new specimen, and retest.
- Read the drug screen test results when control lines are clearly visible.
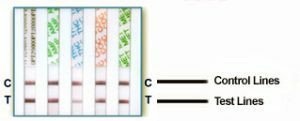
| Interpreting the Test Results | ||
| Negative (-) | Preliminary Positive (+) | Invalid |
|
A negative result is indicated by the presence of both a “C” control line or and a “T” test line for each designated drug. If a color band is visible in each control region "C" and the appropriate drug test region "T", it indicates that the concentration of the corresponding drug of that specific test region is absent or below the detection limit of the test. The presence of a “C” control line and a very light “T” test line indicates a negative result. Any indication of a “T” test line is interpreted as a negative result. There is no meaning attributed to line color intensity or width. Any visible line is considered to be a line. |
A positive result is indicated by the presence of a “C” control line and the absence of a “T” test line. A color band is visible in each control region "C". If no color band appears in the appropriate drug test region "T", a preliminary positive result is indicated for the corresponding drug of that specific test region. |
An invalid result is indicated when the “C” line is completely missing from one or more test windows. If this happens, run another test. If a color band is not visible in the control region "C", the test is invalid. Another test should be run to re-evaluate the specimen. If test still fails, please contact the distributor or the store, where you bought the product, with the lot number. |
 |
||
| SAMHSA Approved CLIA Waived Urine Drug Panel Options Multi-Drug Urine Test Cup is an immunoassay for the qualitative determination of single or multiple drugs in human urine at the cutoff concentrations of table below |
||||
| Drug (Identifier) | Calibrator | Cut-off Level | Minimum Detection Time | Maximum Detection Time |
| Amphetamine (AMP 1000) | d-Amphetamine | 1000 ng/mL | 2-7 hours | 1-2 days |
| Amphetamine (AMP 500) | d-Amphetamine | 500 ng/mL | 2-7 hours | 1-2 days |
| Secobarbital (BAR) [Barbiturates] | Secobarbital | 300 ng/mL | 2-4 hours | 1-4 days |
| Buprenorphine (BUP) | Buprenorphine | 10 ng/mL | 4 hours | 1-3 days |
| Oxazepam (BZO) [Benzodiazapines] | Oxazepam | 300 ng/mL | 2-7 hours | 1-2 days |
| Cocaine (COC 300) | Benzoylecgonine | 300 ng/mL | 1-4 hours | 2-4 days |
| Cocaine (COC 150) | Benzoylecgonine | 150 ng/mL | 1-4 hours | 2-4 days |
| 2-ethylidene-1,5-dimethyl -3,3-diphenylpyrrolidine (EDDP) [Methadone Metabilite] | 2-ethylidene-1,5- dimethyl-3,3- diphenyl- pyrrolidine | 300 ng/mL | 3-8 hours | 1-3 days |
| Methylenedioxymetham- phetamine (MDMA) [Ecstasy] | 3,4-Methylenedi- oxymethamphe- tamine | 500 ng/mL | 2-7 hours | 2-4 days |
| Methamphetamine (MET 1000) [Meth] | D(+)-Methamph- etamine | 1000 ng/mL | 2-7 hours | 2-4 days |
| Methamphetamine (MET 500) [Meth] | D(+)-Methamph- etamine | 500 ng/mL | 2-7 hours | 2-4 days |
| Opiates (OPI 2000) | Morphine | 2000 ng/mL | 2 hours | 2-3 days |
| Morphine (MOP 300) | Morphine | 300 ng/mL | 2 hours | 2-3 days |
| Methadone (MTD) | Methadone | 300 ng/mL | 3-8 hours | 1-3 days |
| Oxycodone (OXY) | Oxycodone | 100 ng/mL | 4 hours | 1-3 days |
| Phencyclidine (PCP) | Phencyclidine | 25 ng/mL | 4-6 hours | 7-14 days |
| Propoxyphene (PPX) | d-Propoxyphene | 300 ng/mL | 2 hours | 2-3 days |
| Nortriptyline (TCA) [Tricyclic Antidepressants] | Nortriptyline | 1000 ng/mL | 8-12 hours | 2-7 days |
| Cannabinoids (THC) [Marijuana] | 11-nor-∆9-THC- 9-COOH | 50 ng/mL | 2 hours | Up to 5+ days |
| 6-Monoacetylmorphine (6-MAM) [Synthetic Heroin] | 6-Monoacetyl- morphine | 10 ng/mL | 2-8 hours | 1-3 days |
| This test is intended for over-the-counter (OTC) use. For in vitro diagnostic use only. The test provides only preliminary results. Clinical consideration and professional judgment should be applied to any drug of abuse test result, particularly in evaluating a preliminary positive result. To obtain a confirmed analytical result, a more specific alternate chemical method is needed. GC/MS or LC/MS is the recommended confirmatory method. | ||||
| Adulteration Control Panels (see configuration above) | |
| Creatinine (CRE) | Daily creatinine excretion, related to muscle mass of the human body, is usually constant. The DOT guideline states that urine specimens with creatinine levels of less than 20 mg/dl are indications of adulteration. Although these ranges are affected by age, sex, diet, muscle mass and local population distribution2, sample with creatinine level of lower than 20 mg/dl should be considered adulterated. |
| Glutaraldehyde (GLU) | Glutaraldehyde is not a natural component of human urine and it should not be present in normal urine. The presence of glutaraldehyde in the urine sample indicates the possibility of adulteration. However, false positive may result when ketone bodies are presence in urine. Ketone bodies may appear in urine when a person is in ketoacidosis, starvation or other metabolic abnormalities. |
| Nitrite (NIT) | Nitrite (NIT): Although nitrite is not a normal component of urine, nitrite levels of up to 3.6 mg/dl may be found in some urine specimens due to urinary tract infections, bacterial contamination or improper storage. In this adulteration control, nitrite level above 7.5 mg/dl is considered abnormal. |
| PH Level (pH) | Normal urine pH ranges from 4.5 to 8.0. Values below pH 4.0 or above pH 9.0 are indicative of adulteration. TEST LIMITATIONS QUESTIONS AND ANSWERS 1. This test has been developed for testing urine samples only. No other fluids have been evaluated. DO NOT use this device to test specimen other than urine. 2. Technical or procedural errors, as well as interfering substances in the urine specimen may cause incorrect results. 3. Contaminated or adulterated urine samples may produce incorrect results. Strong oxidizing agents such as bleach (hypochlorite) can oxidize drug analyte. If a sample is suspected, repeat the test with another urine sample. |
| Specific Gravity (S.G.) | Random urine may vary in specific gravity from 1.003 - 1.030. Normal adults with normal diets and normal fluid intake will have an average urine specific gravity of 1.016 - 1.022. Elevated urine specific gravity value may be obtained in the presence of moderate quantities of protein. DOT guidelines state that a urine specimen with specific gravity level of less than 1.003 is an indication of adulteration. Specific gravity and creatinine values should be considered together to provide a better picture of whether the sample is adulterated. |
| Oxidants (OXI) | The presence of Bleach and other oxidizing reagents in the urine is indicative of adulteration since oxidizing reagents are not normal constituents of urine. Other oxidizing reagents include Hydrogen Peroxide, Ferricyanide, Persulfate, Pyridinium Chlorochromate…etc. |
| Pyridinium Chlorochromate (PCC) | The presence of any chromate in urine is indicative of adulteration as chromate is not a normal constituent of urine. |